Reaction:
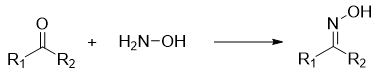
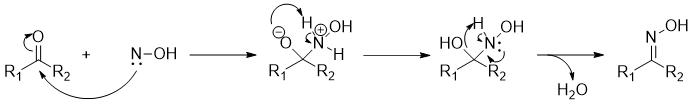
Mechanism [1]:
Specific example [2]:

Highlights and Significance:
- Nitrogen-containing organic compounds are usually in bioactive drugs. Therefore, oximes formation reactions are important to pharmaceutical chemistry. [3]
- Aldehyde and ketone groups can be easily introduced in to varies of compounds without reacting with its other functional groups. This determines it could be widely applied. [4]
- The oximes formation reactions have a high selectivity. [5]
- The products are stable in a wide pH range. [5]
- This reaction is used to produce oligonucleotides conjugates with peptides. [6]
References:
1. Williams, A.; Bender, M. L., Studies on the mechanism of oxime and ketimine formation1. J. Am. Chem. Soc. 1966, 88 (11), 2508-2513.
2. Dirksen, A.; Hackeng, T. M.; Dawson, P. E., Nucleophilic catalysis of oxime ligation. Angew. chem. int. ed 2006, 45 (45), 7581-7584.
3. Dong, J. W.; Ding, T.; Zhang, S. Y.; Chen, Z. M.; Tu, Y. Q., A Facile Approach to Oximes and Ethers by a Tandem NO+‐Initiated Semipinacol Rearrangement and H‐Elimination. Angew. Chem. 2018, 130 (40), 13376-13380.
4. Larsen, D.; Pittelkow, M.; Karmakar, S.; Kool, E. T., New organocatalyst scaffolds with high activity in promoting hydrazone and oxime formation at neutral pH. Org. Lett. 2014, 17 (2), 274-277.
5. Spinelli, N.; Edupuganti, O. P.; Defrancq, E.; Dumy, P., New Solid Support for the Synthesis of 3 ‘-Oligonucleotide Conjugates through Glyoxylic Oxime Bond Formation. Org. Lett. 2007, 9 (2), 219-222.
6. Edupuganti, O. P.; Singh, Y.; Defrancq, E.; Dumy, P., New Strategy for the Synthesis of 3′, 5′‐Bifunctionalized Oligonucleotide Conjugates through Sequential Formation of Chemoselective Oxime Bonds. Chem.: Eur. J. 2004, 10 (23), 5988-5995.